By Marek Vargapal on Sep 12, 2023 1:09:00 PM
What is High Corrosion Protection (HCP) and how to avoid corrosive damage in a steel structure? The surface of steel frames must usually be protected to avoid corrosive damage during the required lifetime. This damage is identified when corrosion in progress jeopardises the functionality of a component or a structural system. Unlike façades that have to meet additional aesthetic requirements, support structures are primarily looked at in terms of their unchanged load capacity throughout their lifetime.
Keep reading this article to have a full explanation of Corrosion Protection and how to achieve it with different types of coatings:
| 1. STANDARD | 2. CUSTOMISED |
What do you need to know to achieve High Corrosion Protection?
Generally, steel components need to be protected against corrosion to guarantee their integrity throughout their planned service life. If corrosion damage occurs and remains unnoticed, it can have a dangerous impact on the components or even the entire system. For load-bearing structures, in particular, it is important to focus on ensuring unrestricted and safe use during the planned service life. To meet this requirement, tender documents and contracts often specify certain coatings or coating systems, even though no specific knowledge of the local atmosphere, micro or macroclimate is available. The latest innovations in surface and coating technology are often unconsidered.
It is therefore vital to gain an overall view of the requirements of the site. Any kind of systematic corrosion protection planning would require analysing the climatic conditions the construction is exposed to.
This should be done according to the European Standard (EN) “Corrosivity categories for atmospheric environment”, or equivalent local regulations. These categories are defined according to EN ISO 12944-2 and reflect six different groups of atmospheric environmental conditions, ranging from “insignificant” up to “extreme”. The norm recommends using these categories and its examples to check the corrosivity at a certain location. The table below illustrates this:
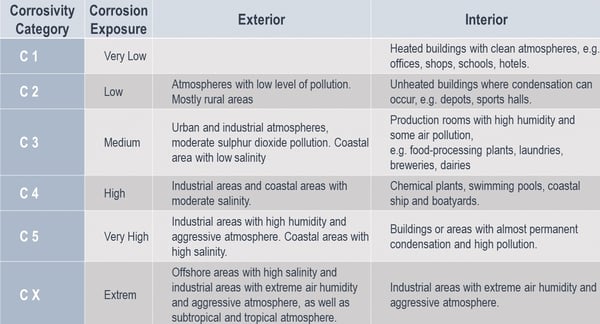
These categories do not take into account any special external influences, such as thermal, chemical, microclimatic, mechanical or construction-related factors, which could shorten the service life of the corrosion protection. These external factors will have high importance when determining the corrosivity category or selecting the right protection.
Coatings are properly assigned to corrosivity categories after carrying out a salt spray test. A certain number of hours in the salt spray without formation of red rust is specified.
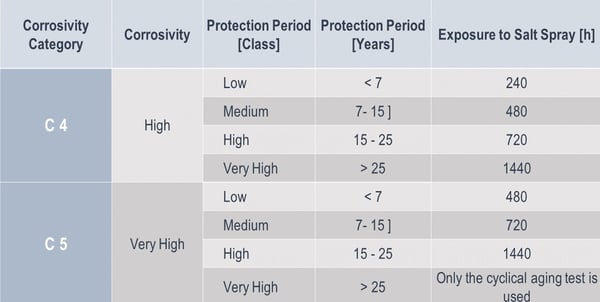
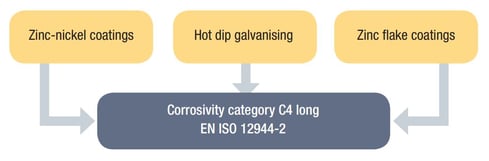
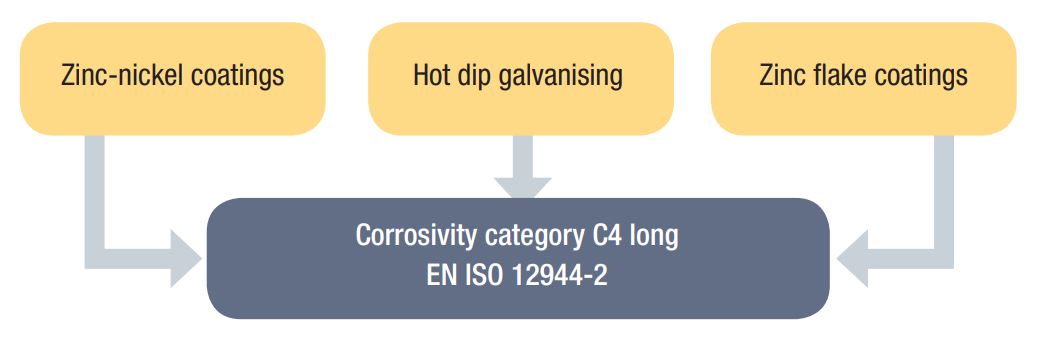
Sikla components with HCP protection system are assigned to corrosivity category C4 long (complying with the requirements of DIN EN ISO 12944-2). The HCP protection system withstands more than 720 hours in the salt spray test without formation of red rust.
Besides the corrosivity categories, which are solely referring to the location of the application, it is also relevant to consider the required lifetime. In order to approach this systematically, the duration has been segmented into different intervals.

For our modular box-section system siFramo 80 with customised multilayer coating, we received a proof certificate confirming compliance with environment category/corrosion risk C5-I (very high), based on a salt spray test duration of 1,500 h. Our protection system is then suitable for the following environmental conditions according to EN ISO 12944 II:
– Outdoor air: very humid industrial atmosphere
– Buildings or areas with permanently present condensation and pollution
1. Standard HCP protection systems
The appropriate anti-corrosion finish for the designated corrosivity category can be achieved through several methods, as examples below:
- Hot Dip Galvanizing - HDG (DIN EN ISO 1461)
A proven and well-known corrosion protection coating, which is used in categories up to C4 and C5. Tenders and projects often specify a minimum layer thickness. Less well-known is that the standard determines and specifies the layer thickness in relation to the thickness of the material being coated. The layer thickness ranges from 45 to 85 µm.
Hot dip galvanising is however unsuitable for more delicate forms (such as small drilled holes or blind hole threads) and may also be unfavourable depending on the design. During the necessary pickling and subsequent drying process, acid residues can remain trapped in joints and gaps. After hot dip galvanising, these residues are not visible and lead very quickly to the formation of red rust and to “rust bleeding” from the gap after exposure to moisture for the first time.
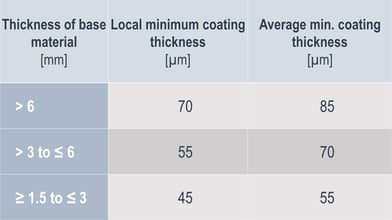 Zinc layer thicknesses according to material thickness (cf. DIN EN ISO 1461:2009-10, Table 3)
Zinc layer thicknesses according to material thickness (cf. DIN EN ISO 1461:2009-10, Table 3)
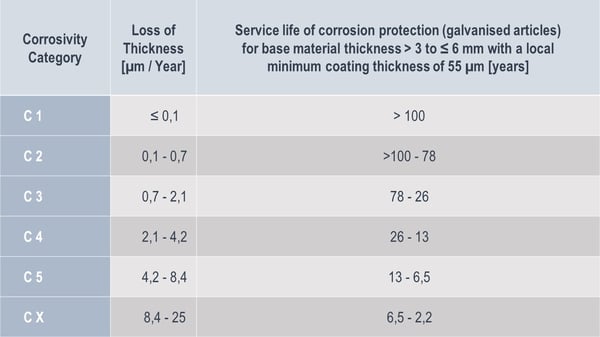 Loss of thickness rates for galvanised articles according to corrosivity category
Loss of thickness rates for galvanised articles according to corrosivity category
(cf. DIN EN ISO 14713-1:2010-05, Table 1)
What about the finishes & appearances of HDG hot-dip galvanized coatings?
It can in fact be affected by several factors. So, to ensure that all the specifications have been met, it occurs a visual inspection, observing surface conditions and checking all contact points, welds, junctions, and bend areas.
Galvanized coatings can take many different appearances, due to several number of reasons: steel chemistry (silicon and phosphorous levels) and cooling rates (thinner material cools faster, staying shinier; thicker pieces stay hot and turn matte grey). What is important to retain is that the corrosion protection level is the same, and that the visual differences will become uniform over time when exposed to natural weather conditions.
- Zinc-Nickel Coatings (DIN EN ISO 19598)
This coating was originally developed for the automotive industry, which has stringent requirements for corrosion protection against exposure to temperature, salt and climatic influences.
Zinc-nickel coatings are applied using a so-called electrolysis method. This involves applying a voltage to a conductive solution containing metal ions, which causes a metallic layer to form on the electrodes. The cathodes used in the electroplating process are the components that require coating.
10 times more corrosion resistance than HDG
The corrosion resistance of zinc-nickel coatings is around 10 times higher than that achieved with hot dip galvanising. For this reason, the layer thicknesses can also be reduced by a factor of 10 (about 8 to 10 µm).

- Zinc Flake Coatings (DIN EN ISO 10683 and DIN EN 13858)
These also have their origins in the automotive industry. They have also been used in the construction industry for quite some time, for protecting components made of high-strength steel (such as screws with strength class >10.9, high-strength nuts, structural parts with tensile strength >1,000 N/mm² etc.). The background to this is the risk of hydrogen embrittlement when using galvanic coating processes.
The layer thickness of 5 to 15 µm is also significantly reduced in comparison to hot dip galvanised components, since the resistance to corrosion is much better. This type of coating is called cathodic protection, whereby the coating “sacrifices” itself to protect the base metal. There is no undermining of the corrosion protection. The excellent properties of this coating system have been tested and confirmed by MPA Stuttgart.

Summing up Sikla standard coating options, this is what you have available:
2. Sikla HCP Customised - for highest demands
For special applications, e.g. outdoors, in coastal areas or aggressive atmosphere, there are higher demands than usual in terms of corrosion protection. Sikla can offer you an individually customised anti-corrosion solution for such applications. Keep reading to learn more about the available options:
- CDP Coating (Cathodic Dip Painting)
- Zinc-lamella Coating (High-performance Protection)
Zinc and aluminium lamellas are applied by dip-spin process and are finally burned-in.
What are the advantages?
- Cathodic protection by ‘sacrifice anode’ of zinc
- Seal effect against oxygen and electrolyte by overlapping zinc and aluminium flakes
- Resistant against organic solvents
- No hydrogen embrittlement
- Thin layers preserve the functionality of small parts, e.g. Self-forming Screw FLS
- Free of Chrome VI and heavy metals
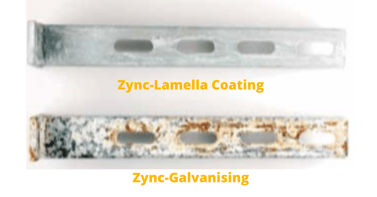 Cantilever Bracket 41/41 after 1,440 h salt spray test.
Cantilever Bracket 41/41 after 1,440 h salt spray test.
- CDP Coating (Cathodic Dip Painting)
The material is dipped in electrically conductive paint. A direct current field is applied between the material and a counter electrode. A closed paint film with excellent adhesive properties is created by the process.
What are the advantages?
- Outright and even coating even in cavities, at corners and edges
- Scratch, impact and hydrochloric acid resistant
- Clean-exhaust painting process
- CDP coated surfaces have ideal characteristics for follow on layers like powder coating
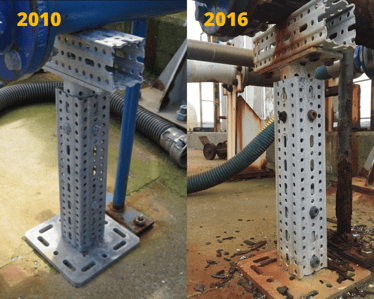
We've conducted a case study (at Lower Hutt, New Zealand), showcasing the Cathodic Protection process associated with our finishing first hand. Check in this image the final comparison:
- Powder Coating
The paint powder is sprayed to the metal part using an electrostatic gun. The object is then heated to 180 °C and the powder melts into a uniform film. It is finally cooled to form a hard coating that has a high bonding and durability performance.
What are the advantages?
- Scratch and impact resistant
- Resistant against chemicals
- Weatherproof
- Free of solvents, environmentally friendly
- Great variety of colours, variable shininess
HCP Protection Systems - Contact us for experienced support!
At Sikla we refer to offer you the most effective corrosion protection.
To select the optimal surface coating for your demands, we attach great importance to the protective effect, the preservation of the functionality (e.g. mobility of the thread), market requirement and economic feasibility. Additionally, our preference is on state-of-the-art technologies that are environmentally friendly and that guarantee the most efficient and comfortable installation performance.
Should you need further guidance, our team will find with you the most suitable surface coating, providing experienced support and advice!
Contact us for more information!

Written by Marek Vargapal
Business Development Engineer (Energy from Waste & Power Generation)


comments